How ELAHERE may help you be here
Patient portrayal.
ELAHERE may help stop cancer from progressing
In a clinical study of 453 people with FRα+ platinum-resistant ovarian cancer, ELAHERE helped extend the time to disease progression compared with traditional chemotherapy.
Define:
FRα=folate receptor alpha.
Median progression-free survival

(227 people)
Traditional
chemotherapy
(226 people)
More time without the cancer growing or spreading with ELAHERE
A chance for better response is here
The study also found that more people’s tumors shrank by at least 30% or disappeared when treated with ELAHERE vs when treated with traditional chemotherapy. The decrease in tumor size lasted a median of 6.8 months in those treated with ELAHERE and 4.5 months in those treated with traditional chemotherapy.
Overall response*
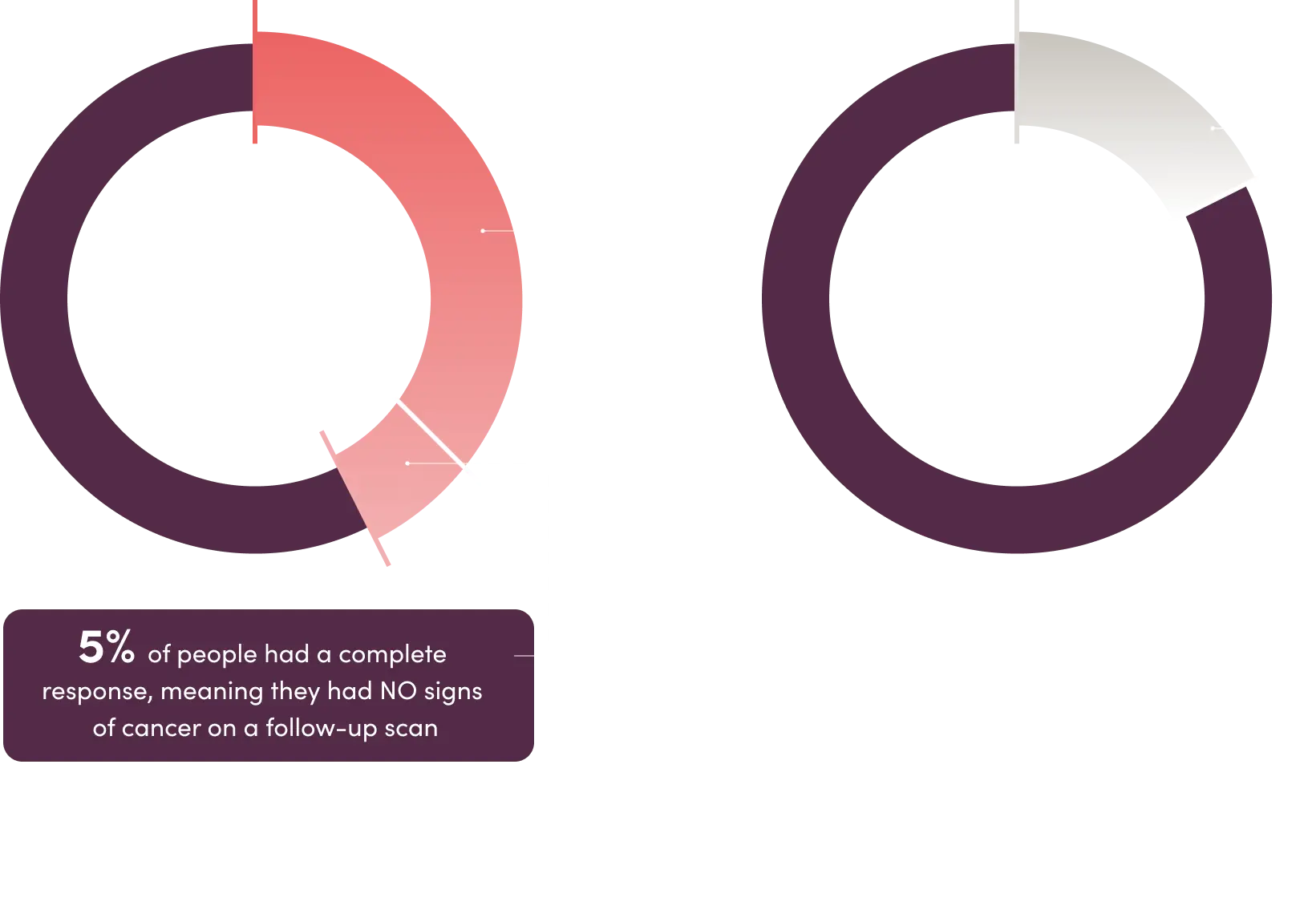
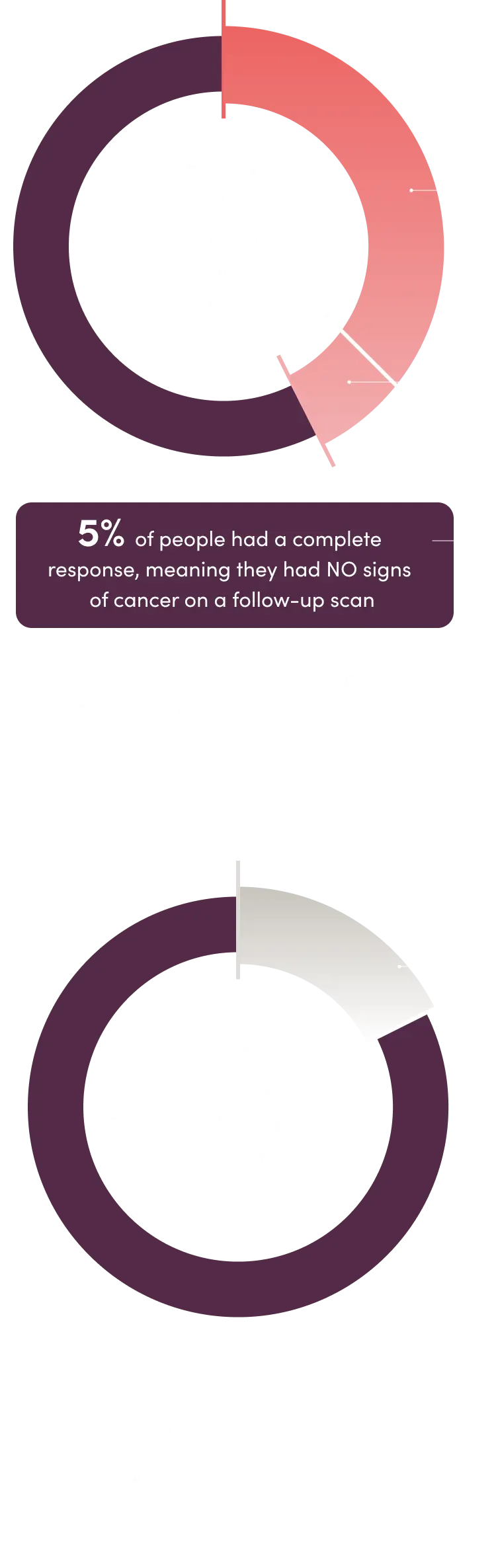
*In the clinical study, 225 people treated with ELAHERE and 224 people treated with traditional chemotherapy were eligible to be evaluated for overall response.
Define:
Curious about side effects?
Learn about the possible side effects of treatment with ELAHERE.
Talk with your doctor about ELAHERE
For help starting a conversation with your doctor, personalize this discussion guide.
Stay up-to-date with ELAHERE
Receive helpful resources and treatment tips right in your email inbox.